A 28-year-old man was admitted after CPR due to ventricular fibrillation and in incipient cardiogenic shock based on newly diagnosed dilated cardiomyopathy. Past medical history was insignificant.
Physical examination and laboratory findings on admission:
- jugular venous distension, pedal edema and bibasal crackles on lungs;
- blood pressure: 90/70 mmHg;
- brain natriuretic peptides (BNP) 2549,5 ng/l.
ECG (on admission): sinus rhythm, heart rate 93 bpm, PQ 200ms, QRS 128ms, QTc 497ms, right axis deviation, flattened T in I, aVL, negative T in V6, rS V1-V5
Chest radiograph (on admission): enlargement of cardiac silhouette with markedly prominent left cardiac contour, enlarged hilar vessels without increased peripheral pulmonary vascular markings
Transthoracic and transoesophageal echocardiography showed the following findings:
- severe left ventricular dilatation (EDD 94 mm) and severe diffuse hypokinesis of the left ventricle (LV) with reduced ejection fraction (LV EF <20%) (without thrombi);
- left atrial dilatation (LAVi 52 cm3/m2) with no thrombi
- dilated right ventricle with moderate to severe systolic dysfunction (fractional area change - FAC 26%);
- right atrial dilatation;
- mitral regurgitation (3/4) caused by mitral annular dilatation and tethering of mitral leaflets (coaptation depth CD 21mm, AP 41mm, IC 40 mm, ERO 0,33)
- tricuspid regurgitation (2/4, PG 43 mmHg), estimation of PASP 53-58 mmHg;
- pulmonary regurgitation (3/4), dilatation of main pulmonary artery 41 mm;
- signs of pulmonary hypertension
- atrial septal defect secundum type (13x15-17 mm, area 3D 1,4cm2) with left-to-right shunt (In this case, an echocardiographic calculation of Qp:Qs is not reliable because of moderate to severe pulmonary regurgitation.)
Video 1 TTE examination on admission

Video 2 TEE examination showing ASD

Video 3 TEE confirming ASD

Image 1 Size of ASD

Cardiac MR finding corresponded to dilated cardiomyopathy, late gadolinium enhancement was present in the septal area.
Coronary angiography showed no significant coronary artery stenosis.
Right heart catheterization was performed with following findings.
- moderate postcapillary pulmonary hypertension, mPAP 38 mmHg, PAR 0,7 WU, elevated RV filling pressure (RV EDP 23 mmHg);
- low cardiac output (CO 3,94 l/min a CI 1,52 l/min/m2).
- large left-to-right shunt (Qp:Qs 2,7:1);
Saturation in aorta - 96%
Saturation in left atrium - 95%
SVC - 51%
IVC - 58%
PA - 79%
RV - 79%
CT angiography was subsequently performed and ruled out anomalous pulmonary venous return.
During hospitalization, the patient had a good response to milrinone. Initially, small doses of inotropes were needed. In the further course of hospitalization, the patient was hemodynamically stable without inotropic and vasopressor support. The pharmacological treatment of congestive cardiac failure was prescribed (ACE inhibitor, beta-blocker, aldosterone antagonist, diuretics, ivabradine). ICD implantation was indicated and performed for secondary prevention of sudden cardiac death.
Closure of the atrial septal defect was not indicated because of the severe LV dysfunction. The patient was placed on the waiting list for orthotopic heart transplantation.
Due to early need for re-hospitalization for signs of low cardiac output he was put on an urgent waiting list of HTx and successfully underwent transplantation 4 months after initial episode of heart failure.
CLINICAL CONTEXT AND LEARNING POINTS
Dilated cardiomyopathy (DCM) is characterized by dilation of one or both ventricles with impaired systolic function and may or may not develop overt heart failure. DCM is caused by a variety of disorders, although frequently no etiology can be found and the cardiomyopathy is deemed idiopathic. Causes of DCM include stress-induced, infectious, toxic, genetic, peripartum, tachycardia-mediated cardiomyopathy, sarcoidosis, end-stage kidney disease, autoimmune disease, endocrine dysfunction, and nutritional deficiencies. Current major society classification systems for cardiomyopathy exclude heart disease secondary to coronary artery disease, valvular or congenital heart disease.
Atrial septal defect (ASD) is the most common congenital heart lesion in adults and is often asymptomatic until adulthood. ASDs in adults are associated with left-to-right shunt causing volume overload of the right heart chambers and pulmonary arteries with possible late development of progressive pulmonary vascular obstructive disease and pulmonary hypertension. The severity of the shunt is determined by the size of the defect and atrial and ventricular compliance and pressure.
There are four major types of atrial septal defects (Image 2):
- Secundum ASD (70 - 75 % of all ASDs) is a defect in the septum primum resulting from poor growth of the secundum septum or excessive absorption of the septum and is usually located in the region of the fossa ovalis and its surrounding.
- Primum ASD (15 - 20 % of ASDs) is a defect in the septum secundum caused by failure of the primum septum to fuse with the endocardial cushions at the base of the interatrial septum. This type is prevalently associated with anomalies of the atrioventricular valves with or without a defect in the ventricular septum.
- Sinus venosus defects account for 5 to 10 % of ASDs and are located in the venoatrial portion of the atrial septum. Sinus venosus defects represent an abnormality in the insertion of the superior or inferior vena cava. An anomalous connection involving one or more pulmonary veins is frequently present with sinus venosus ASD.
- Unroofed coronary sinus is caused by absence of the common wall between the coronary sinus and the left atrium. This defect accounts for less than 1 % of ASDs.

Jean Marie Carabuena. Atrial septal defect. Consults in Obstetric Anesthesiology pp 75-76.
METHODS OF CARDIAC SHUNT QUANTIFICATION
Qp:Qs RATIO USING INVASIVE OXIMETRY
Invasive oximetry using Fick’s principle for the quantification of left-to-right shunting is based on measurements of blood oxygenation. Interventional closure or corrective surgery is usually indicated in patients with a Qp:Qs ratio >1.5.
Percentage oxygen saturation is required from the following: SVC, IVC, RA, RV, PA, PCW, aorta x2
* An increase in O2 blood saturation can be detected, typically an increase of more than 7% suspect a shunt
Mixed venous (MV) blood = (3xSVC + 1xIVC) / 4
* Blood saturation in IVC is affected by low blood desaturation in the kidneys
Formula:

Ao - aortic, Mv - mixed venous, Pv - pulmonary veins, Pa - pulmonary artery
Since pulmonary veins are rarely entered during a cardiac cath, a pulmonary catheter wedge sample or LA sample (if the LA is entered through the ASD) can be used in its place !!! Alternatively, arterial saturation can be substituted !!!

Image 3. 'Oximetry run' in a patient with atrial septal defect. The 'step-up' detected in the right atrium (RA) identifies a left-to-right shunt at this location.

https://www.pcipedia.org/wiki/Hemodynamic_principles:_pressure_measurement,_cardiac_output_and_shunt_detection
Qp:Qs RATIO USING DOPPLER ECHOCARDIOGRAPHY
The volume of fluid moving via a tube may be calculated by multiplying the cross-sectional area (CSA) by the velocity at which the fluid is moving. Thus, one may use CSA of the left and right ventricular outflow tracts (RVOT and LVOT) and respective summated average velocities during systole to determine the flow through the right and left circulations. Outflow tract diameters are obtained in the parasternal short and long axis, respectively. Pulsed wave Pulse doppler through the outflow tracts yields a spectral envelope which one may trace and yield a velocity time integral (VTI).
Formula: Qp:Qs= CSA RVOT x RVOT VTI(CSA LVOT) x (LVOT VTI)
Where to measure (Image 4)?

Oakley L, Foley S, Cox J, et alAn adult with a sinus venosus atrial septal defect and dilated cardiomyopathyCase Reports 2014;2014:bcr2013201306.
Suspicion on cardiac shunt already from right heart cath
Signs of recirculation should be investigated on transpulmonary thermodilution measurements of cardiac output (Image 5).

https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20821/transpulmonary-thermodilution-cardiac-output-measurement
ASD closure in patients with severe dysfunction of LV
Concomitant LV dysfunction causes an increase in LV end-diastolic and left atrial (LA) pressure. In the setting of an ASD, this results in increased left to right shunting and provides a means of “offloading” the less compliant left ventricle. Abrupt closure of this interatrial communication in the setting of transcatheter closure results in an acute increase in left ventricular and left atrial filling pressures as well as myocardial oxygen consumption and may lead to acute decompensation of the left ventricle and pulmonary oedema.
Due to aforementioned reasons, our patient was not indicated for ASD closure due to expected deterioration after the procedure. Patient was indicated for heart transplant.
REFERENCES
- Baumgartner, H., De Backer, J., Babu-Narayan, S. V., Budts, W., Chessa, M., Diller, G.-P., lung, B., Kluin, J., Lang, I. M., Meijboom, F., Moons, P., Mulder, B. J. M., Oechslin, E., Roos-Hesselink, J. W., Schwerzmann, M., Sondergaard, L., Zeppenfeld, K., Ernst, S., … Ladouceur, M. (2020). 2020 ESC Guidelines for the management of adult congenital heart disease. European Heart Journal, 42(6), 563–645. https://doi.org/10.1093/eurheartj/ehaa554
- Sanders, S. P., Yeager, S., & Williams, R. G. (1983). Measurement of systemic and pulmonary blood flow and QP/QS ratio using doppler and two-dimensional echocardiography. The American Journal of Cardiology, 51(6), 952–956. https://doi.org/10.1016/s0002-9149(83)80172-6
- Gatzoulis, M. A., Swan, L., Therrien, J., & Pantely, G. A. (Eds.). (2005). Adult Congenital Heart Disease. Wiley. https://doi.org/10.1002/9780470750544
- Carroll, D., Knipe H. et al. Cardiovascular shunts. Radiopaedia. Retrieved July 04, 2021, from https://radiopaedia.org/articles/cardiovascular-shunts?lang=us
- Naser M Ammash, MD, Heidi M Connolly, MD, FACC, FASE, Susan B Yeon, MD, JD, FACC (2020). Clinical manifestations and diagnosis of atrial septal defects in adults. UpToDate. Retrieved July 02, 2021, from https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-atrial-septal-defects-in-adults?search=atrial%20septal%20defect&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2
- Marilyn Weigner, MD, FACC, James P Morgan, MD, PhD, William J McKenna, MD, Todd F Dardas, MD, MS (2019). Causes of dilated cardiomyopathy. UpToDate. Retrieved July 09, 2021, from https://www.uptodate.com/contents/causes-of-dilated-cardiomyopathy?search=dilated%20cardiomyopathy&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- https://www.pcipedia.org/wiki/Hemodynamic_principles:_pressure_measurement,_cardiac_output_and_shunt_detection
- Jean Marie Carabuena. Atrial septal defect. Consults in Obstetric Anesthesiology pp 75-76.
- https://journals.lww.com/cardiologyinreview/Fulltext/2019/05000/Thermodilution_Cardiac_Output__A_Concept_Over_250.5.aspx
Authors: Michal Pazderník, Erik Schmotzer
You Might Also Like
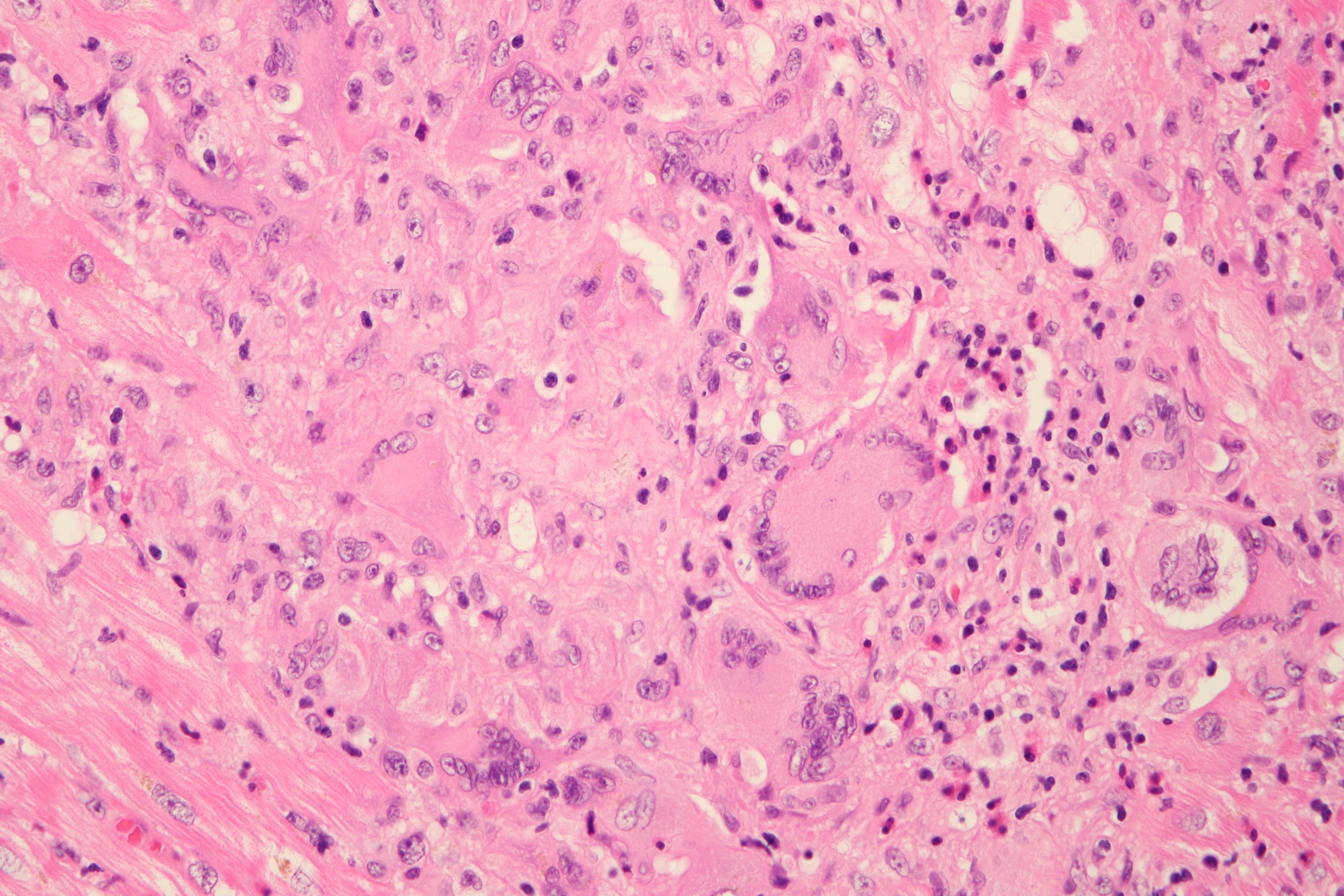
Cardiogenic shock due to Giant Cell Myocarditis
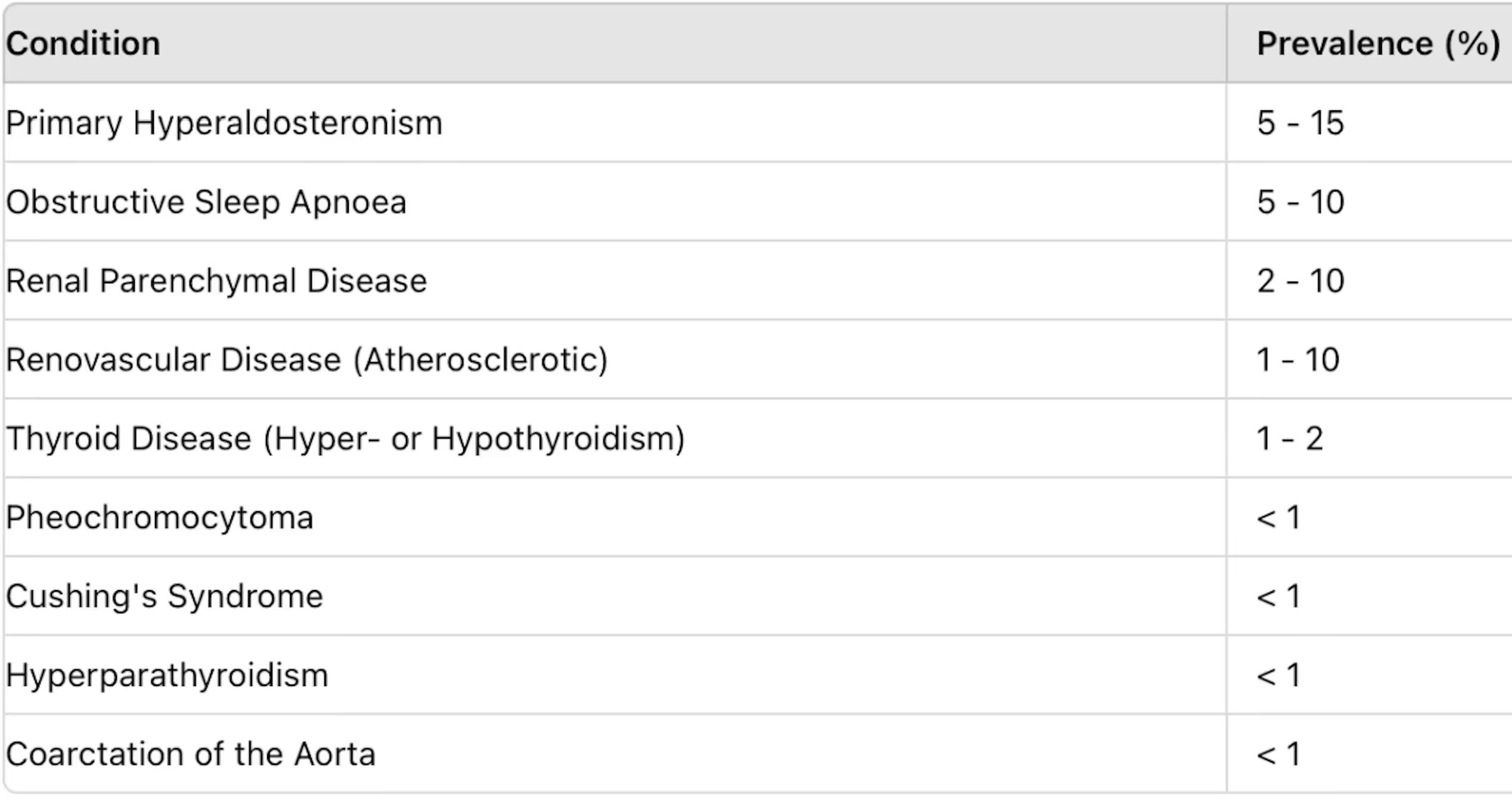
Secondary hypertension


.jpg)


